Data Analysis
- Click "Import results" to import the qPCR data. Select the correct file.
- KMRengine will perform quality analysis on the data and present the data in the plate view.
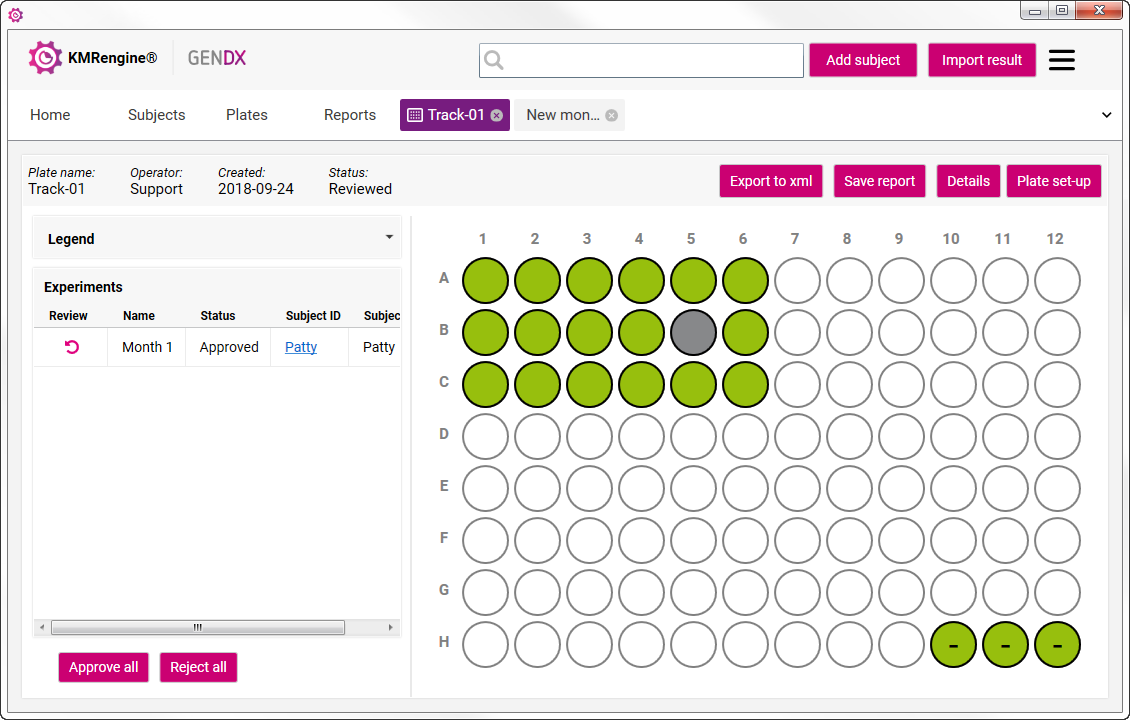
- KMRengine analyzes replicates for precision.
- Wells turn green when replicate groups qPCR values differ by less than 2% CV.
- Wells turn red when replicate groups display a variance greater than 2% CV.
- Wells turn yellow when the result is uninterpretable (e.g. when the reference of either the Pre or the Post sample is not correct).
- By double-clicking on the plate, the data window expands and shows the qPCR data.
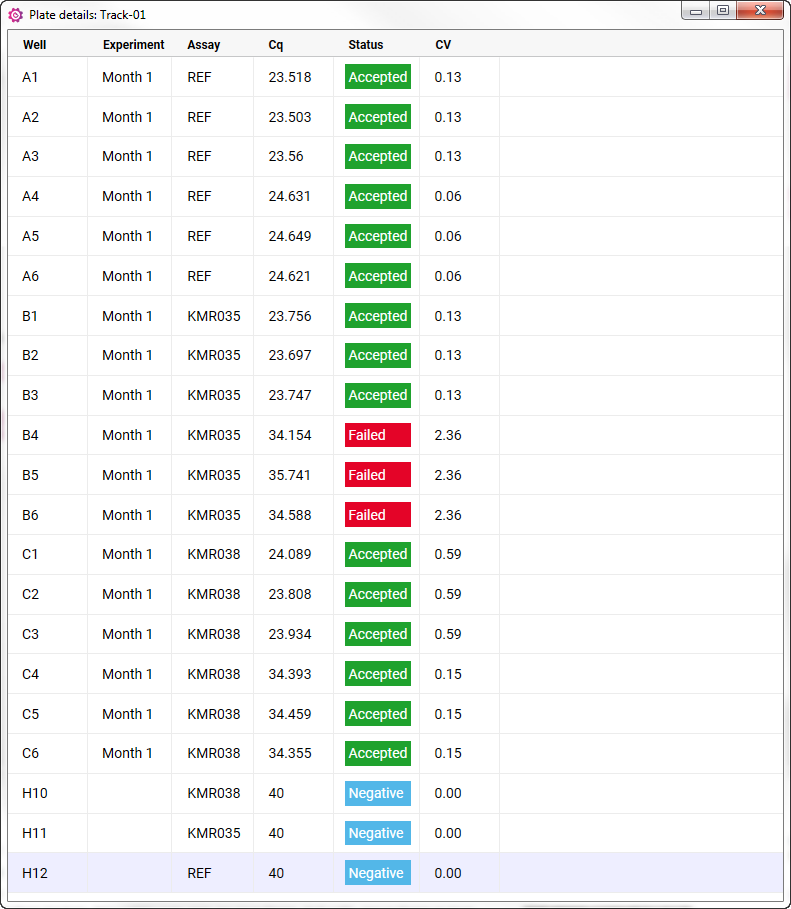
- Data can be inspected to decide whether all replicates can be accepted, or whether some need to be rejected.
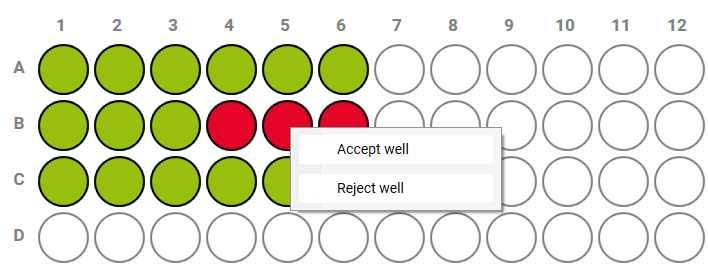
- Accept or reject data by right-clicking on a cell or well.
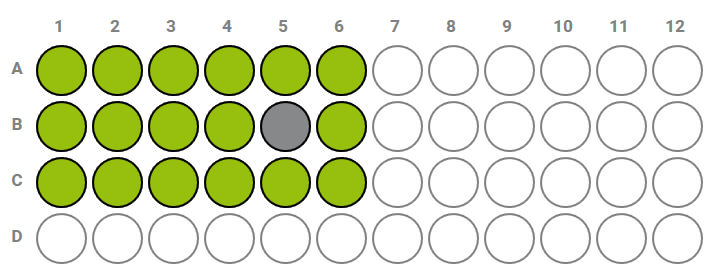
- Rejecting the deviating data point in a replicate series causes the well to turn gray and the remaining wells to turn green.
- After reviewing the data, the result can be approved or rejected:
 Approve the result for this sample
Approve the result for this sample
 Reject the result for this sample
Reject the result for this sample
 Undo review
Undo review
Via the "Approve all" or "Reject all" buttons, all samples can be approved or rejected simultaneously.
- Click "Save report" to obtain the tracking report.
- Click "Export to xml" to export an xml file for this experiment.
- Go to the Subjects tab and click on the Report icon behind the Recipient to open the Monitoring report:
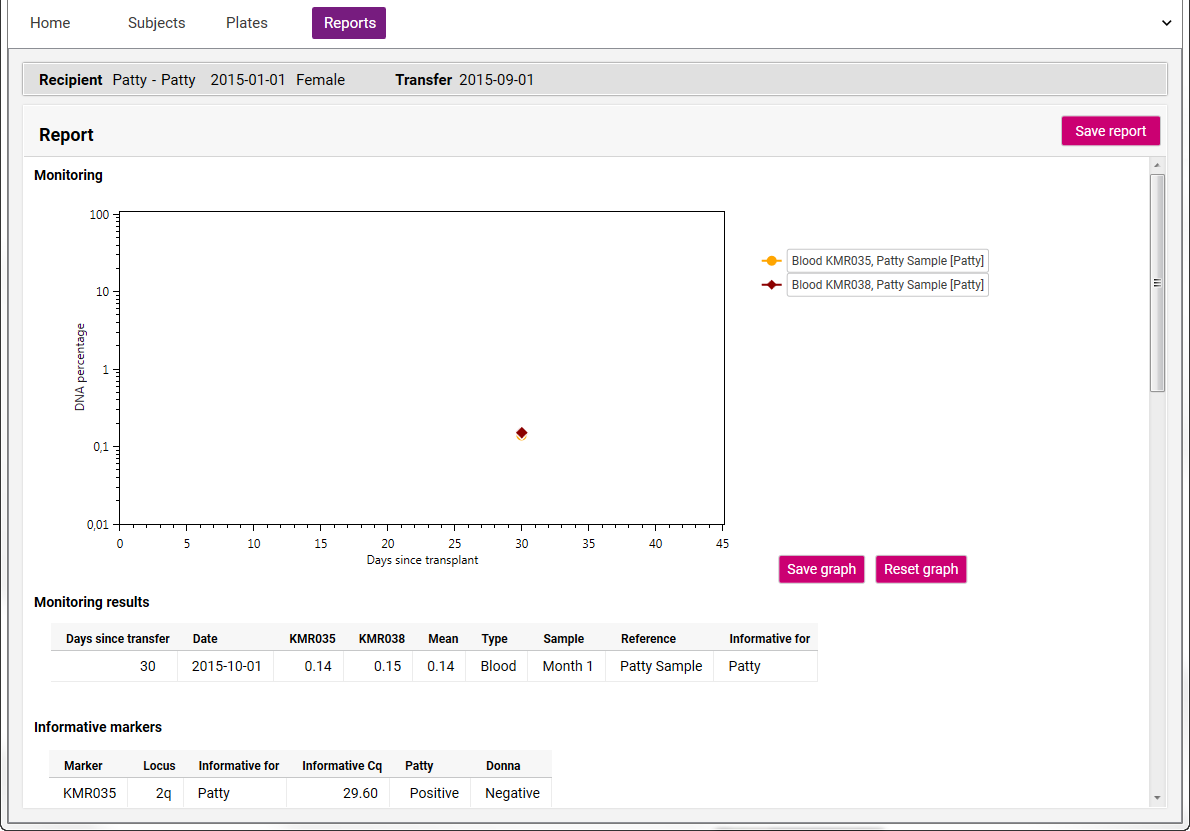
- The report displays a graph with the chimerism percentages over time.
- This graph can be saved to the clipboard or as an image (PNG file) via the "Save graph" button.
- By scrolling on the graph, the scaling can be made larger or smaller. To go back to the default graph setting, click on the "Reset graph" button.
As more data is collected for a particular sample over time, KMR engine provides a longitudinal view.
- To view the composite set of data for an individual sample, move to the Subjects tab and select the "Open report" hyperlink of a recipient.