Companion Diagnostic solutions with GenDx: Your partner from start to finish
Our Companion Diagnostics (CDx) services are designed to facilitate and support the pharmaceutical precision medicine revolution. New pharmaceutical therapies in the field of precision medicine require the presence of specific biomarkers or genetic profiles, which impact the eligibility or susceptibility of patients for the pharmaceutical therapy. For example, the genetic HLA profile of a patient can determine the response of a patient to a particular pharmaceutical therapy (read more here). The continuous progress in the field of precision medicine goes hand in hand with an increasing need for companion diagnostics that allow accurate patient selection. GenDx, as a strong CDx partner, will support clinical study validation, ease navigating regulatory landscapes, and contribute to the success of a pharmaceutical therapy.
At GenDx, we do everything in-house, from Research & Development to Regulatory Affairs ensuring an efficient and seamless process. In May 2022, GenDx became the first company in the field of transplantation diagnostics to attain IVDR certification for their HLA typing reagents and analysis software. Our analysis software NGSengine is recognized as the best-in-class solution for the analysis of NGS-based HLA typing, ensuring accuracy and reliability in analyzing one of the most complex and heterogenous gene families.
We offer our cutting-edge technology and extensive expertise in molecular diagnostics for complex, polymorphic genes, such as immuno-related genes like HLA, KIR, and MICA/MICB. Together with our evidencable experience with IVDR, GenDx will be your knowledgeable and reliable partner in supporting the development of your CDx, organization of clinical performance studies, and registration of new CDx products.

With Companion Diagnostics
Targeted patient identification
Identify patients most likely to benefit from your therapy
Risk stratification
Identify individuals at increased risk for serious adverse reactions
Therapy monitoring
Monitor treatment response for optimized safety and efficacy

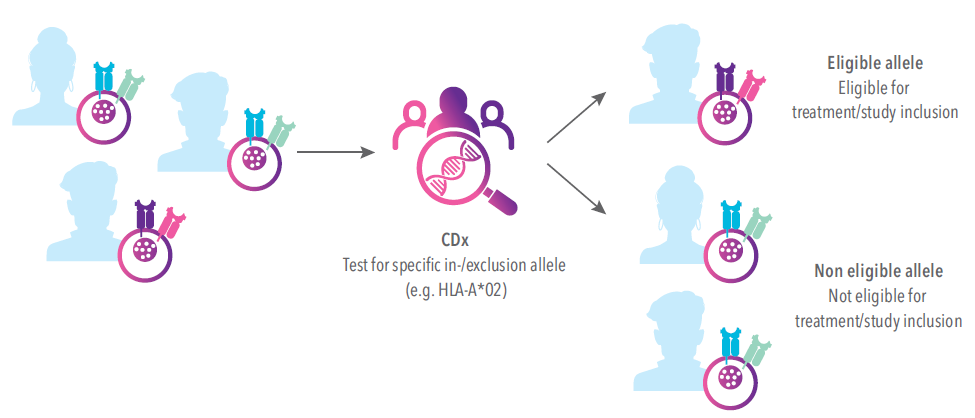
What we offer
With expertise in molecular diagnostics and regulatory compliance, GenDx ensures a fast, efficient, and reliable CDx development process. We provide tailor-made CDx solutions aligned with your therapeutic strategy, offering:
✔ CE-marked molecular diagnostics under IVDR – NGS-based HLA typing reagents and analysis software
✔ Rapid development timeline – From CE-marked legacy device to tailor-made clinical performance study assay in 6-9 months
✔ Broad assay development capabilities – Experience with developing diagnostic solutions for full gene analysis of complex, polymorphic genes, such as HLA, KIR, MICA/MICB and many more
✔ End-to-end expertise – Assay development including, manufacturing, supply chain and regulatory support
✔ Full-service CDx support – Facilitating bridging studies and interventional studies from idea to CDx
✔ Proven regulatory experience – Europe and USA approvals, with global expansion capability
✔ Flexible, collaborative approach – Tailored solutions to meet your CDx needs
✔ Strong global network – Supporting clinical studies & regulatory submissions

Our portfolio
Our broad portfolio of products and analysis techniques enables the precise analysis of complex genes. We provide state-of-the-art molecular diagnostics based on:
- Next-Generation Sequencing (NGS) – long- and short-read, PCR-based or capture-based enrichment, single locus or multiplex, library preparation, and sequence platform agnostic analysis software
- Sanger Sequencing – Reagents and analysis software for high-resolution Sanger sequencing-based HLA typing
- qPCR and dPCR – cfDNA (cell free DNA) and gDNA (genomic DNA) analysis & software

Our development strategy
Our dedicated project team will work closely with you to ensure that every aspect of the project aligns with your specific requirements.



Meet the Team


